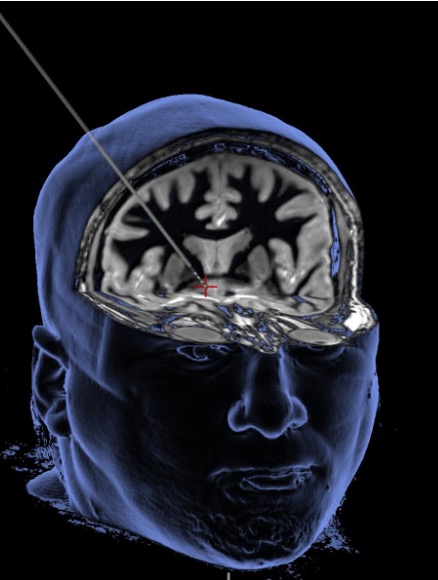
Deep brain stimulation delivers a mild electrical current through a thin wire to alter brain activity in the brain’s reward center (see red plus sign).
Image credit: WVU Rockefeller Neuroscience Institute
Repeated drug use changes the brain, which is one reason addiction is so hard to overcome. Over a period of weeks to months, use of opioids and other substances alter brain circuits as the body adapts to the effects of these chemicals.
Yet, with repeated drug use over time, the body may need more and more drug to feel the same effect. People with drug addiction often continue to use drugs to avoid the harrowing physical and psychological withdrawal symptoms from stopping drugs abruptly – creating a vicious cycle that is hard to break.
The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative® recognizes that multiple strategies are needed to address all aspects of the addiction lifecycle. That means identifying risk factors, stopping overdoses, developing effective treatments, and promoting stable recovery. For each of these aspects, technology can be a powerful tool.
In a particularly innovative approach, HEAL-funded neurosurgeon Ali Rezai, M.D., of the West Virginia University Rockefeller Neuroscience Institute in Morgantown is evaluating the use of deep brain stimulation for patients with severe, treatment-resistant opioid use disorder.
Resetting the Brain
The human brain is a complex and dynamic labyrinth of interconnecting circuits. It allows us to sense the world around us as well as respond to changing conditions. In turn, the brain adapts.
“An individual is not destined to be a person with addiction, but with continued drug use they become that person over time,” Rezai explains. The brain gets “rewired” to create changes in the brain’s reward circuit deep in the brain where dopamine is released in response to rewarding stimuli like drugs.
With repeated drug use, however, dopamine production drops off, and craving intensifies. More and more drug is needed to produce a high. The reward circuit sends signals to another part of the brain, the prefrontal cortex, which helps control behavior and is important for decision making and working toward goals.
Overstimulation of the reward center and understimulation of the behavior control center create a difficult combination: the desire and physiological need to use more drugs and less impulse control to resist. In this scenario, it is very difficult for people who try to quit drugs.
Rezai is leading a HEAL research study to help change brain circuits whose activity underlies drug addiction, using focused electrical energy from an implanted device. Called deep brain stimulation, the procedure is sort of like a pacemaker for the brain that can restore a healthy electrical activity and rhythm. Over the past 30 years, neurosurgeons like Rezai have inserted more than 200,000 deep brain stimulation implants into patients with a range of neurologic disorders.
Stimulating Hope
Deep brain stimulation delivers a mild electrical current to a specific brain region to alter its activity, tuning it up or down. The technique has been used successfully to treat Parkinson’s disease, where it acts like and on-off switch for tremors characteristic of this debilitating movement disorder. Deep brain stimulation is also used to treat epilepsy and obsessive-compulsive disorder, and researchers are testing deep brain stimulation for several other conditions including chronic pain, depression, and Alzheimer’s disease.
Rezai’s HEAL research is now testing deep brain stimulation as part of a strategy for opioid use disorder that has not responded to other treatments. Rigorously designed experiments are critical for determining whether this technology, in combination with other treatments like medication and counseling, can reset the brain and lead to reduced drug use. Although some studies have been done in China and Europe, Rezai’s is the first to be designed, controlled, and regulated by the U.S. Food and Drug Administration to determine how well this approach works.
Participants in Rezai’s deep brain stimulation study have chronic, severe opioid use disorder. These individuals (four, so far) have experienced multiple overdoses and typically have not responded to all of the treatments they have tried: inpatient, outpatient, or sober housing approaches. Participating in this research study represents a big leap of hope for recovery.
Determining if the approach is safe is the current goal. In the coming months, 20 more patients will receive an implant toward bringing their brain back into balance. If deep brain stimulation proves to be effective for stabilizing their brain rhythms as planned, the approach might offer a life-changing opportunity for people who have exhausted other options.
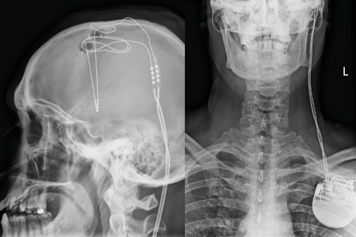
The deep brain stimulation wire (left) is connected to a pacemaker in the chest (right) that can both stimulate and record electrical activity.
Image credit: WVU Rockefeller Neuroscience Institute
Although the human brain is only about 3 pounds, it is packed with 86 billion neurons and billions of other cells, including those that make up blood vessels – all encased in a protective bone structure. Finding the precise spot to target with a stimulating contact and electrode is tricky.
This deep brain stimulation procedure takes about 6 hours, during which Rezai threads a tiny 1 mm-diameter brain lead with four electrode contacts through a nickel-sized hole in the skull and down to brain structures that are part of the reward circuit. The brain wire is connected to a pacemaker in the chest that can both stimulate and record electrical activity. Only the research team can tune up or down electrical stimulation of brain within study participants.
Magnetic resonance imaging guides neurosurgeons like Rezai to be able to see the reward structures in a participant’s brain, and a computer modeling program uses those images to create a 3D map of the brain circuit. Because those patterns are highly unique among brain regions, they can be used to distinguish circuits right next to each other that perform completely different functions. Thus, the implant can be placed precisely where it needs to go.
Perhaps the most important help, though, comes from the person on the operating table, who is awake and can respond to cues (like being shown a picture of drug injection). The research team measures reactions to these cues that cause changes in brain activity both to guide placement of the implant and to calibrate the device for each patient. Because the brain and skull are not able to sense pain, and the scalp has been numbed in advance, the procedure does not hurt.
Throughout the study, Rezai and his research team offer 24/7 support to all participants and can tweak stimulation as needed. For example, one individual called over a weekend and was struggling with severe anxiety. When the researchers took another look at their brain imaging maps and the location of the implant, they knew why: too much stimulation to a nearby brain region linked to fear and emotions.
“So we moved the stimulation higher up,” Rezai explains, to get closer to the prefrontal cortex, “and his symptoms dramatically improved.”
Beyond Surgery
In the search for lasting treatments for opioid addiction, a person-centric focus is necessary to accommodate individuals on their personal road to recovery. Most people need a range of strategies, including medications, counseling, and other support services. All of these are continued after an implant is placed within an individual’s brain to deliver stimulation treatment.
Another important goal of this research is to better understand how reward and behavior pathways change with craving, relapse, and recovery. Rezai and his team are looking for electrical signatures of brain changes that affect behavior: “We’re working on ways to continuously monitor addiction pathways in the brain wirelessly.”
As a first step to finding such signatures, the scientists use the implanted electrodes to measure electrical activity in the brains of people participating in the study. In time, as these paths become clearer, deep brain technology could be able to automatically sense and deliver therapy to optimize electrical signals and restore healthy rhythms.
Although the main goal of this research study is to determine safety, the team is cautiously optimistic from early results and thrilled for the improved health of the small group of participants who have undergone the procedure.
“Our first patient celebrated sobriety and complete abstinence for 3 years. He used to relapse every other week,” Rezai says, adding that another has been entirely abstinent for more than a year and is now working as a peer recovery coach.
“They go from severe, end-stage addiction to becoming therapists themselves … that’s really amazing.”
Stay Connected
Stay up to date on the latest HEAL Initiative research advances by subscribing to receive HEAL content directly to your inbox.
Read More About This Program
Read about the research program, Focusing Medication Development to Prevent and Treat Opioid Use Disorder and Overdose.
National Institute on Drug Abuse (NIDA)
Learn more about NIDA's role in the NIH HEAL Initiative.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
